
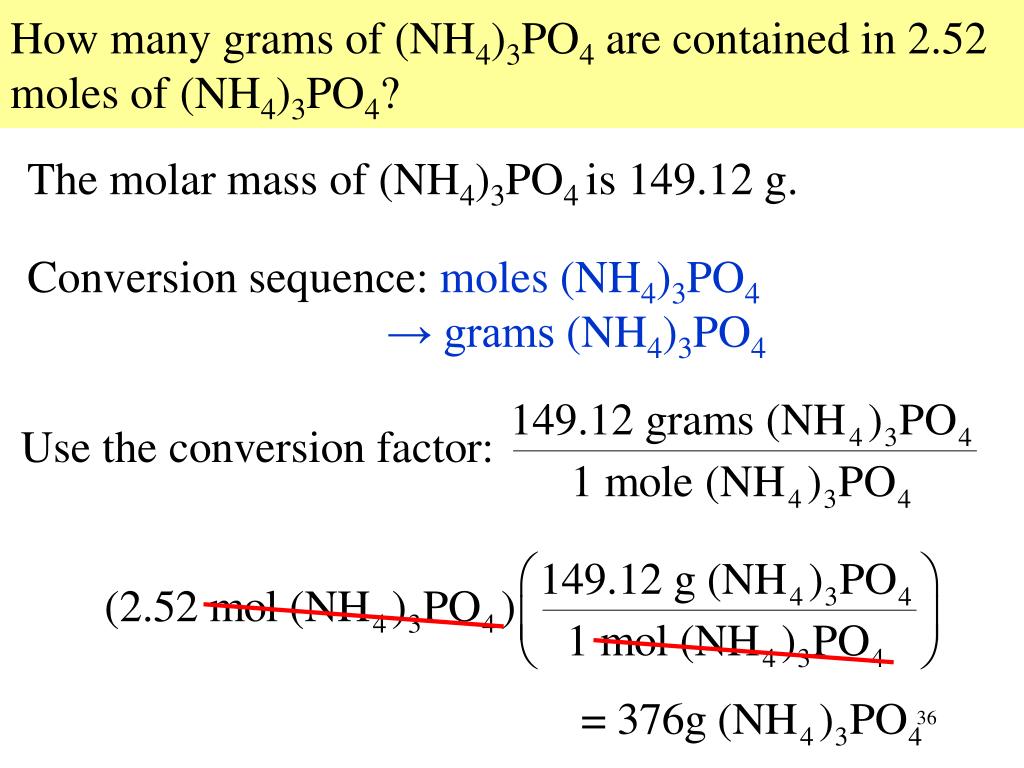
Molar Mass Of O2 (Oxygen Molecule)Īn element’s molecular mass is defined as the sum of the masses of its constituent elements. Therefore, the formula mass of calcium hydrogen carbonate is 117.10 amu and the molar mass of calcium hydrogen carbonate is 117.10 grams per mole (g/mol). If we are talking about a mole of an ionic compound, however, we will still use the term molar mass. Ionic compounds do not have individual molecules. When referring to compounds that are not molecular (ionic compounds), the term “molecular mass” is inappropriate, and “formula mass” is generally used instead. In other words, N2 has a molar mass of 28.02 grams per mole. This result is called the molecular mass of a molecule. As for nitrogen, its atomic mass is simply (14.01 + 14.01) = 28.02 amu.Īn element’s atomic mass is simply the sum of the atomic masses of all its constituent atoms. For a molecule (for example, nitrogen, N2), the mass is equal to the sum of the atomic masses of the two nitrogen atoms. Compounds can also be measured using the concept of molar mass. Understanding what molar mass is and how it is related to doing calculations in chemistry will help us understand why knowing oxygen’s molar mass is important. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License. We recommend using aĪuthors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Use the information below to generate a citation. Then you must include on every digital page view the following attribution: If you are redistributing all or part of this book in a digital format, Then you must include on every physical page the following attribution: If you are redistributing all or part of this book in a print format, Want to cite, share, or modify this book? This book uses the If the molecular (or molar) mass of the substance is known, it may be divided by the empirical formula mass to yield the number of empirical formula units per molecule ( n):
As the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass.
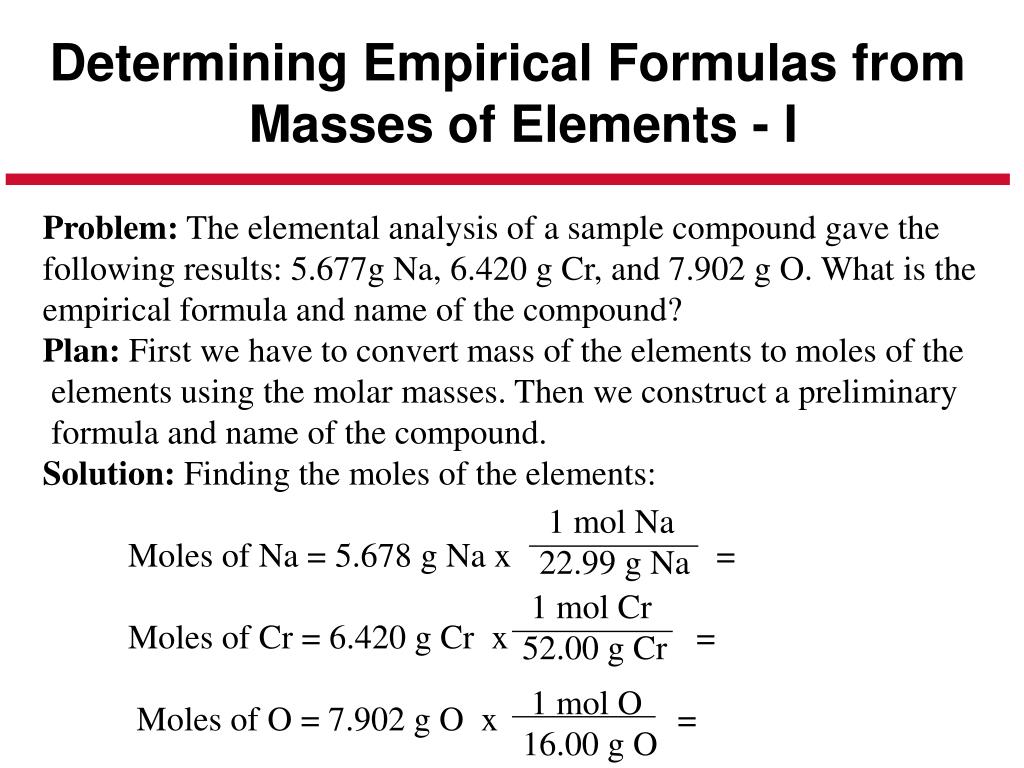
Molar mass can be measured by a number of experimental methods, many of which will be introduced in later chapters of this text. Molecular mass, for example, is often derived from the mass spectrum of the compound (see discussion of this technique in the previous chapter on atoms and molecules). These quantities may be determined experimentally by various measurement techniques. Determining the absolute numbers of atoms that compose a single molecule of a covalent compound requires knowledge of both its empirical formula and its molecular mass or molar mass. Recall that empirical formulas are symbols representing the relative numbers of a compound’s elements. The percent composition of this compound could be represented as follows: For example, consider a gaseous compound composed solely of carbon and hydrogen. The results of these measurements permit the calculation of the compound’s percent composition, defined as the percentage by mass of each element in the compound. When a compound’s formula is unknown, measuring the mass of each of its constituent elements is often the first step in the process of determining the formula experimentally. The elemental makeup of a compound defines its chemical identity, and chemical formulas are the most succinct way of representing this elemental makeup. But what if the chemical formula of a substance is unknown? In this section, these same principles will be applied to derive the chemical formulas of unknown substances from experimental mass measurements. Given the chemical formula of the substance, one may determine the amount of the substance (moles) from its mass, and vice versa. The previous section discussed the relationship between the bulk mass of a substance and the number of atoms or molecules it contains (moles). Determine the molecular formula of a compound.Determine the empirical formula of a compound.Compute the percent composition of a compound.

By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
